Tess Hatch hopes to travel to space, in the meantime she is a venture investor at
Bessemer Venture Partners, investing in frontier tech.
More posts by this contributor
We have airplanes and drones in our airspace and satellites in space, but what about the space in between: the stratosphere?
There are platforms, such as blimps, balloons and high-altitude long endurance (HALE) fixed-wing platforms that can duplicate functions now performed by drones or satellites in a more technically and commercially viable manner.
Commercial drones operate in our airspace below 400 feet. Commercial aircraft fly between 9-12km (30,000-39,000 feet). Satellites operate in low Earth orbit (LEO, 500-1200km), mid Earth orbit (MEO, 2000-36,000km) and geostationary Earth orbit (GEO, 36,000km).
But what about the vast space in between our air space and LEO? The approximately 488km of space known as the stratosphere, is, at present, largely uninhabited and underutilized.
The problem
Imagine if a platform wants to loiter over a single point on the Earth for an extended period of time, either to maintain situational awareness and consistent surveillance over an area of interest or maintain communications. For example, after a natural disaster, it would be invaluable and life saving to have eyes, ears and a voice in the sky monitoring and helping the afflicted. Or what if the platform were able to monitor a natural disaster before it made landfall to collect better data on the storm’s size, location and path?
Other reasons why it might be advantageous to have persistent real-time video from the sky is surveillance of vast maritime regions and borders, identification of objects of interest and monitoring events, including storms, fires and environmental disasters, on behalf of first responders and enforcement agencies.
Another example could be global internet connectivity. If platforms mesh together and talk to one another, they could connect the world below in a much more effective and efficient manner than ground-based fiber optic cables. It could monitor our oceans or protect vulnerable people from exploitation. And the potential military, intelligence and governmental applications are obvious and substantial.
In short, the applications are abundant and the potential market for this type of platform massive.
Possible existing solutions
Right now, the prevalent existing airborne platforms are drones and planes, and the prevalent existing space-based platforms are satellites. Each platform has various benefits, but none are optimized for many of the missions described above and, thus, do not necessarily accomplish those missions in the most efficient and effective manner.
Quadcopter drone
- Pro: Cheap, close to the ground
- Con: Can fly for only on average 30 minutes (unless you are using Impossible Aerospace’s US-1 that has over two hours of flight time), needs access to the ground below, small field of view from 400 feet, can be easily detected
|
 Image Credits: Impossible Aerospace |
Uncrewed plane
- Pro: Larger field of view from 30,000 feet
- Con: Can fly for only the number of hours fuel is available, expensive, can be detected
|
|
Constellation of LEO satellites
- Pro: Large field of view from 500-1,200km
- Con: One would need hundreds or thousands of satellites in orbit for full-world coverage since the 90-minute orbits have access only over a single point on the Earth for ~15 minutes of the ~90 minute orbit; also, the satellite needs to be successfully launched from a rocket, escape Earth’s velocity and operate for years in the radioactive vacuum of space (which, while easier and less expensive than a GEO satellite, still requires a fair amount of effort and expense)
|
 Image Credits: Spire Global |
GEO satellite
- Pro: Covers one-third of the Earth
- Con: Large (school-bus sized), expensive (many millions of dollars), takes years (sometimes decades) to design/build/launch and does not provide the necessary low resolution or short latency
|
|
The solutions above are optimized for other types of critical missions. For example, drones are great for monitoring crops or inspecting infrastructure (as Drone Deploy software enables) or delivering emergency medical supplies (which Zipline and Google Wing are doing). Remotely-operated planes like General Atomics MQ-1 Predator have offensive military applications.
Constellations of LEO satellites in space, like Spire Global, can provide maritime, aviation and weather monitoring and prediction, or take photos of the world, as Planet Labs does. Lastly, GEO satellites can also be used for monitoring weather, communication and surveillance, but at a high level, not localized.
Possible future solutions
There are a handful of companies working on solutions specifically optimized for the mission of loitering over a single point. These solutions include balloons, blimps and HALE (high-altitude long endurance) platforms in the stratosphere.
Balloons

Image Credits: WorldView
Companies like Loon, WorldView and WindBorne use air currents in the stratosphere to loiter over a single point. Their platforms have no propulsion on board and the structure consists of two balloons, a lift and a ballast balloon. The lift balloon contains either helium or hydrogen and is sealed with special UV-coated material. They use a compressor to add or remove air from the ballast balloon so that it becomes lighter or heavier to make the balloon go up or down depending on wind speed and direction and which air current they would like to ride.
- Pro: You cannot see these balloons from the ground with the naked eye or with most types of current ground-based tracking systems. They are fairly cheap, can be launched easily and can loiter over a single area for days or even months at a time.
- Con: Without propulsion, balloons are difficult to navigate through intense stratospheric winds, so it might be hard to precisely navigate and keep the balloons over the specific area of interest. The balloons are not recoverable when the flight terminates, although when the balloon bursts and returns to Earth you might be able to recover the payload.
Blimps
- Pro: They are fairly large so they can carry heavier payloads and provide more power to the payload. You can re-land the entire platform to either fix or recover the payload, and launch it multiple times.
- Con: They can be seen from the ground because they are so large, which makes them vulnerable to being shot down. Companies like Sceye and Altaeros are using the Goodyear Blimp with some tech upgrades. Their airships either have propulsion or are tied to the ground below, so they can better control where they are going, and they have upgraded UV and ozone-resistant skin.
HALE fixed-wing
Companies like Zenith and Skydweller are working on high-altitude long endurance (HALE) fixed-wing platforms. These high-aspect-ratio aircraft (which means long but slender wings) are powered by sunlight hitting the solar panels on the wings. The power that is generated can either power the plane and payload or be stored in the batteries. Therefore, if enough power is generated and stored during the day to last throughout the night, the plane can fly indefinitely.
- Pro: They can be precisely controlled by a pilot.
- Con: They have limited power for the payload, as most of the power generated is needed to power the aircraft.
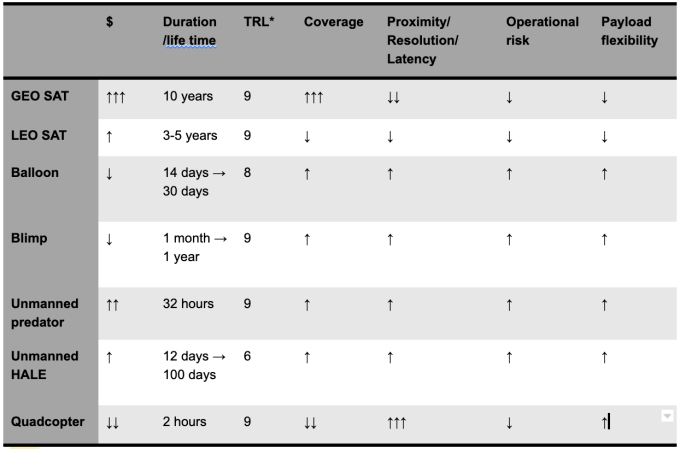
*TRL: technology readiness level
For all of these platforms, there will be additional challenges in the areas of manufacturing and mission management. The platforms need to be manufactured and launched cheaply, quickly and reliably. This takes time and money. Additionally, there are issues relating to who will monitor the platforms once they are in the stratosphere — the company that built the platform or the customers whose payload the platform is holding?
Another issue that platforms that operate in the stratosphere will face relates to who regulates the stratosphere. Obviously, putting and operating platforms in the stratosphere raises a number of regulatory and legal questions that will have to be resolved.
I believe there is enough room in this market (and certainly in the stratosphere) for all of these platforms to be successful. They complement existing platforms such as drones and satellites and, for certain critical missions, can be more effective and efficient than their counterparts that operate in the airspace or in LEO/GEO.










